
3,3"-Difluoro-5,5"-bis(trimethylstannyl)-2,2"-bithiophene is used for the synthesis of small molecules or polymer semiconductors in organic electronic applications, such as P(NDI2OD-T2F).
It is believed that, by introducing heteroatoms such as fluorine on the backbone of polymer structures, the crystalline properties of the conjugated polymers can be enhanced, resulting in a higher degree of orientation of such polymer structures with optimised domain size in blended thin films with either fullerene or non-fullerene acceptors.
This product has been used in our own lab by Ossila chemists for the synthesis of PBDD4T-2F.
General Information
| CAS number | 1619967-09-7 |
| Chemical formula | C14H20F2S2Sn2 |
| Molecular weight | 527.86 g/mol |
| Synonyms | (3,3"-Difluoro-[2,2"-bithiophene]-5,5"-diyl)bis(trimethylstannane), DFBT-bisSn |
| Classification / Family | Bithiophene, Thiophene, Heterocyclic five-membered ring, Organic semiconducting materials, Semiconductor synthesis, Low band-gap polymers, OFETs, OLED, Organic photovoltaics, Polymer solar cells |
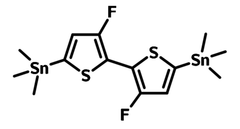
Chemical Structure
Product Details
| Purity | >98% |
| Melting point | n.a. |
| Appearance | White flakes/crystal/powder |
MSDS Documentation
 DFBT-bisSn MSDS Sheet
DFBT-bisSn MSDS Sheet
Literature and Reviews
- Effect of Alkyl Side Chains of Conjugated Polymer Donors on the DevicePerformance of Non-Fullerene Solar Cells, D. Xia et al., Macromolecules, 49 (17), 6445–6454 (2016); DOI: 10.1021/acs.macromol.6b01326.
- High-Performance Non-Fullerene Polymer Solar Cells Based on a Pair of Donor–Acceptor Materials with Complementary Absorption Properties, H. Lin et al., Adv. Mater., 27, 7299–7304 (2015); DOI: 10.1002/adma.201502775.
- Controlling Energy Levels and Blend Morphology for All-Polymer Solar Cells via Fluorination of a Naphthalene Diimide-Based Copolymer Acceptor, M. A. Uddin et al., Macromolecules, 49 (17), 6374–6383 (2016); DOI: 10.1021/acs.macromol.6b01414.
- A Fluorinated Polythiophene Derivative with Stabilized Backbone Conformation for Highly Efficient Fullerene and Non-Fullerene Polymer Solar Cells, S. Zhang et al., Macromolecules, 49 (8), 2993–3000 (2016); DOI: 10.1021/acs.macromol.6b00248.
- Implication of Fluorine Atom on Electronic Properties, Ordering Structures, and Photovoltaic Performance in Naphthobisthiadiazole-Based Semiconducting Polymers, K. Kawashima et al., J. Am. Chem. Soc., 138 (32), 10265–10275 (2016); DOI: 10.1021/jacs.6b05418.
- Over 11% Efficiency in Tandem Polymer Solar Cells Featured by a Low-Band-Gap Polymer with Fine-Tuned Properties, Z. Zheng, Adv. Mater., 28, 5133–5138 (2016); DOI: 10.1002/adma.201600373.
To the best of our knowledge the technical information provided here is accurate. However, Ossila assume no liability for the accuracy of this information. The values provided here are typical at the time of manufacture and may vary over time and from batch to batch.



