
Methylammonium bromide (MABr) is a precursor for the synthesis of organic-inorganic hybrid perovskites for use in FETs, LEDs and PVs.
General Information
| CAS number | 6876-37-5 |
| Chemical formula | CH6BrN |
| Molecular weight | 111.97 g/mol |
| Synonyms |
|
| HOMO / LUMO | n.a. |
| Classification / Family | Organic photovoltaics, Light-emitting diodes, Perovskite precursor materials |
Product Details
| Purity | 98% (M572) >99.5% (M571 further purified by recrytalisation of M572) |
| Melting point | 296 °C |
| Appearance | White crystals/powder |
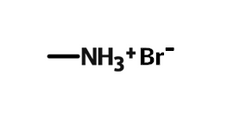
Applications
Methylammonium bromide (MABr) is a precursor of MAPbBr3 perovskites. Having a band gap of 2.3 eV (HOMO 5.68 eV, LUMO 3.38 eV) [1], MAPbBr3 perovskites have been used to tune the band gap of mixed MAPbX3 (where X is the halide I, Br and/or Cl mixtures) [2,3,4,5,6]. For this reason, bromide MAPbBr3 perovskites can be utilised as light absorbers for high-energy photons, and can serve as the front cell in tandem cells. This perovskite can provide a higher open-circuit voltage in perovskite solar cells than the iodide analogue.
High-efficiency solar cells, with a VOC of up to 1.40 V, a fill factor (FF) of 79%, and a PCE of 6.7% have been reported for pure MAPbBr3 perovskite solar cells [1].
It has also been demonstrated that MAPbBr3 nanoplatelets can be employed in light-emitting diodes, exhibiting bright photoluminescence (PL) at 529 nm, with a narrow spectral band and a quantum yield up to 85% [7].
| Device structure | FTO/TiO2/(FAPbI3)0.85(MAPbBr3)0.15/PTAA/Au [8] |
| Jsc (mA cm-2) | 23.3 |
| Voc (V) | 0.94 |
| FF (%) | 65 |
| PCE | 14.2 |
MSDS Documentation
 Methylammonium bromide MSDS sheet
Methylammonium bromide MSDS sheet
Pricing
| Grade | Order Code | Quantity | Price |
| 98% purity | M572 | 10 g | £106.00 |
| 98% purity | M572 | 25 g | £203.00 |
| >99.5% purity | M571 | 5 g | £103.00 |
| >99.5% purity | M571 | 10 g | £169.00 |
| >99.5% purity | M571 | 25 g | £328.00 |
Note: Looking for a bulk order (100 g or more)? Please contact us for a quote.
Literature and Reviews
- Voltage output of efficient perovskite solar cells with high open-circuit voltage and fill factor, S. Ryu et al., Energy Environ. Sci., 7, 2614–2618 (2014). DOI: 10.1039/c4ee00762j.
- Efficient Planar Perovskite Solar Cells Based on 1.8 eV Band Gap Ch2Nh2PbI2Br Nanosheets via Thermal Decomposition, Y. Zhao et al., J. Am. Chem. Soc., 136 (35), 12241–12244 (2014). DOI: 10.1021/ja5071398.
- High Open-Circuit Voltage Solar Cells Based on Organic–Inorganic Lead Bromide Perovskite, E. Edri et al., J. Phys. Chem. Lett., 4 (6), 897–902 (2013). DOI: 10.1021/jz400348q.
- Preparation of Single-Phase Films of Ch2Nh2Pb(I1-xBrx)3 with Sharp Optical Band Edges, A. Sadhanala et al., J. Phys. Chem. Lett., 5, 2501-2505 (2014). dx.doi.org/10.1021/jz501332v.
- Chemical Management for Colorful, Efficient, and Stable Inorganic-Organic Hybrid Nanostructured Solar Cells, J-H. Noh et al., Nano Lett., 13, 1764-1769 (2013). dx.doi.org/10.1021/nl400349b.
- Maximizing the emissive properties of Ch2Nh2PbBr3 perovskite nanoparticles, S. Gonzalez-Carrero et al., J. Mater. Chem. A, 3, 9187-9193 (2015). DOI: 10.1039/C4TA05878J.
- Bright Light-Emitting Diodes Based on Organometal Halide Perovskite Nanoplatelets, Y. Ling et al., Adv. Mater. 2015, DOI: 10.1002/adma.201503954.
- Compositional engineering of perovskite materials for high-performance solar cells, N. Jeon et al., Nature 517, 476–480 (2015), doi:10.1038/nature14133.
To the best of our knowledge the technical information provided here is accurate. However, Ossila assume no liability for the accuracy of this information. The values provided here are typical at the time of manufacture and may vary over time and from batch to batch.



